Select the structure of the conjugated diene – Conjugated dienes, a class of organic compounds characterized by alternating single and double bonds, possess unique properties that set them apart from other dienes. This comprehensive guide delves into the intricate world of conjugated dienes, exploring their resonance structures, isomerism, and reactivity, providing a solid foundation for understanding their behavior and applications.
The distinct structural features of conjugated dienes give rise to remarkable stability and reactivity, making them versatile building blocks in various chemical processes. Their ability to undergo a wide range of reactions, including Diels-Alder reactions and cycloadditions, has made them indispensable in the synthesis of complex organic molecules.
Overview of Conjugated Dienes
Conjugated dienes are a class of organic compounds that contain two double bonds separated by a single bond. This unique structure gives conjugated dienes distinct properties compared to other dienes.
The alternating double and single bonds in conjugated dienes allow for the delocalization of electrons, which results in increased stability and unique chemical reactivity. Conjugated dienes are commonly found in natural products and are used in a variety of industrial applications.
Resonance and Delocalization in Conjugated Dienes
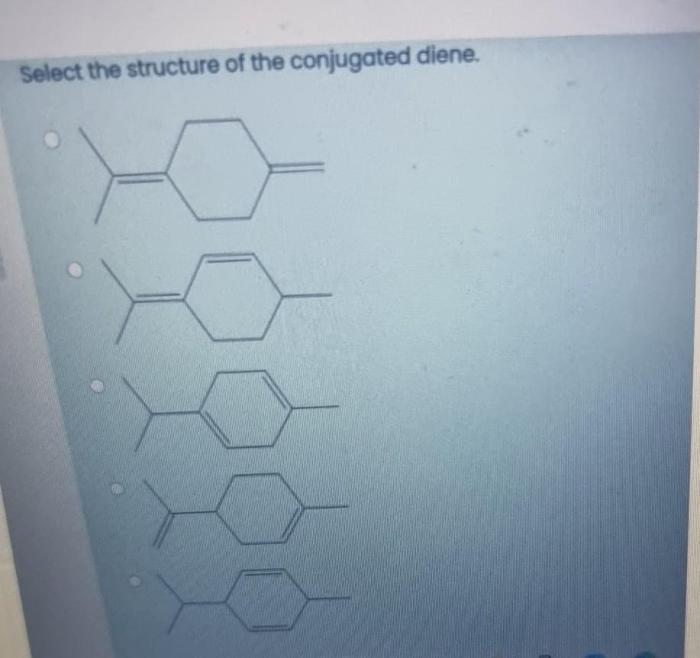
The delocalization of electrons in conjugated dienes is a key factor in their stability and reactivity. Resonance structures can be drawn to represent the different ways in which the electrons are delocalized.
The delocalization of electrons results in a lowering of the overall energy of the molecule, making conjugated dienes more stable than isolated dienes. Additionally, the delocalized electrons are more reactive, which makes conjugated dienes more susceptible to electrophilic addition reactions.
Isomerism in Conjugated Dienes
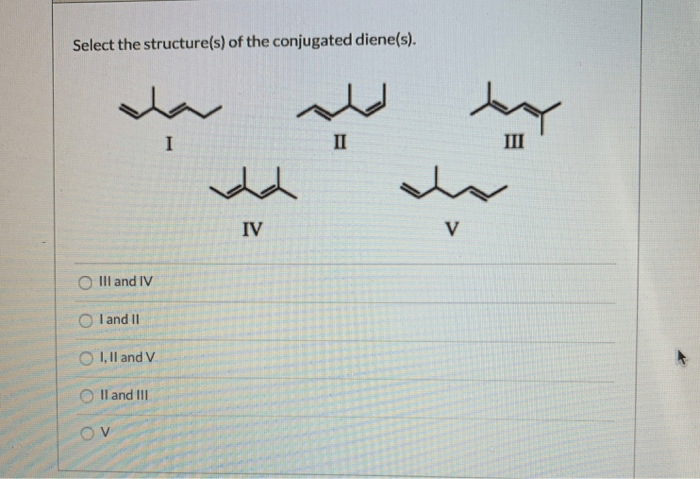
Conjugated dienes can exist as different isomers, depending on the relative orientation of the double bonds. Geometric isomerism occurs when the two double bonds are oriented in the same or opposite directions, resulting in cis-trans isomers.
Conformational isomerism occurs when the two double bonds are rotated around the single bond, resulting in different conformations. The different isomers of conjugated dienes have different physical and chemical properties.
Reactions of Conjugated Dienes: Select The Structure Of The Conjugated Diene
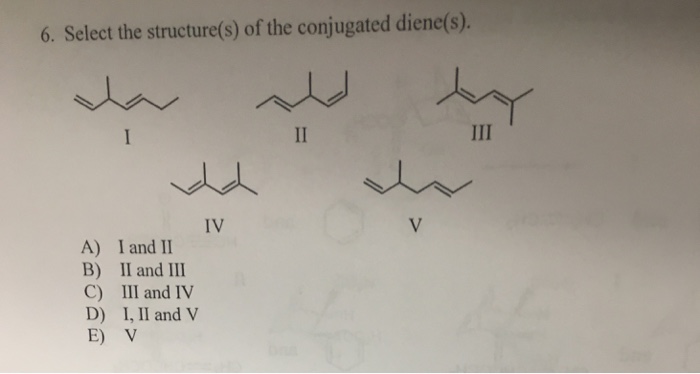
Conjugated dienes undergo a variety of reactions, including Diels-Alder reactions, cycloadditions, and electrophilic additions. These reactions are all characterized by the participation of the delocalized electrons in the conjugated diene system.
Diels-Alder reactions are cycloaddition reactions that involve the reaction of a conjugated diene with a dienophile. Cycloadditions are reactions that involve the formation of a new ring. Electrophilic additions are reactions that involve the addition of an electrophile to the conjugated diene system.
Applications of Conjugated Dienes
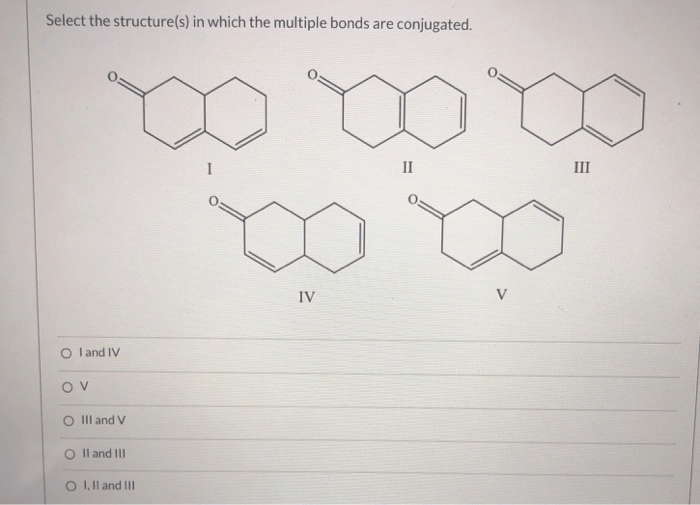
Conjugated dienes are used in a variety of industrial applications, including the synthesis of polymers, pharmaceuticals, and dyes.
Polymers are long chain molecules that are made up of repeating units. Conjugated dienes are used in the synthesis of polymers such as polyethylene and polypropylene. Pharmaceuticals are drugs that are used to treat diseases. Conjugated dienes are used in the synthesis of pharmaceuticals such as ibuprofen and naproxen.
Dyes are compounds that are used to color materials. Conjugated dienes are used in the synthesis of dyes such as azo dyes and anthraquinone dyes.
Clarifying Questions
What are the key structural features of conjugated dienes?
Conjugated dienes are characterized by alternating single and double bonds, resulting in a continuous chain of p-orbitals that allows for resonance and electron delocalization.
How does resonance contribute to the stability of conjugated dienes?
Resonance in conjugated dienes leads to the formation of multiple resonance structures, which distribute the electron density over several atoms, enhancing the overall stability of the molecule.
What types of isomerism are observed in conjugated dienes?
Conjugated dienes exhibit both geometric isomerism (E/Z isomers) and conformational isomerism (s-cis/s-trans isomers) due to the restricted rotation around the double bonds.
What are some common reactions involving conjugated dienes?
Conjugated dienes undergo a variety of reactions, including Diels-Alder reactions, cycloadditions, and electrophilic additions, which exploit their reactivity at the double bonds.